Central Imaging
Banook has dedicated the past 25 years to becoming a global leader in medical imaging technology and associated services. Whether you are a large pharma or biotech company, a CRO, or an academic institution, we can help you with your central imaging needs.

THERAPEUTIC AREAS & INDICATIONS

WORKFLOW
Key components of a central imaging workflow
Our team provides expertise on your imaging protocol design and tailors the reading scheme to your specific imaging endpoints. We will work with you on the following key documents:
Imaging Manual: Outlines the processes to follow for site qualification, optimal image acquisition by sites, and quality control of the imaging data.
Imaging Review Charter: details the independent central read procedure of study images, including the management of central readers, the reading methodology and its rationale, and the reading schedule.
Dosimetry Documentation: describes dosimetry equipment calibration procedures, specific imaging acquisition requirements, and how to analyse the data for dosimetry analysis.

EXPERTISE
Central imaging reader group
Our global panel of 500+ board-certified experts—each with over 10 years of experience—ensures accurate, specialized readings across therapeutic areas. Experts are radiologists, oncologists, neuroradiologists, nuclear medicine physicians, dermatologists and cardiologists based all over the world.
Our scientific team selects and manages central readers to ensure low variability and minimize bias throughout the study.
We ensure reading consistency through statistical monitoring, consensus sessions, regular re-training, and phantom-based bias checks for quantitative imaging.

READING CRITERIAS
Accurate imaging starts with the right reading criteria
We have supported over 350 imaging studies, many in oncology—including pivotal trials—giving us extensive expertise in the following reading criteria:
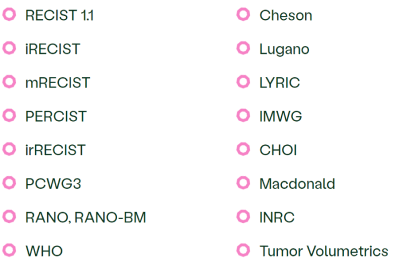
We leverage our expertise to guide sponsors in selecting and implementing the most effective criteria to ensure high-quality, consistent data that supports confident decision-making.

CENTRAL IMAGING MODALITIES
Choosing the right image modality is crucial for achieving trial objectives
We can help you with trials involving all imaging modalities including:

We collaborate closely with clinical teams during the protocol definition phase to ensure that all imaging challenges are addressed.
CENTRAL IMAGING IN CLINICAL TRIALS
Why is central imaging important in clinical trials?
Central imaging is crucial in clinical trials as it ensures consistency in image acquisition and interpretation across multiple sites. By standardizing processes, it reduces variability, minimizes reader bias, and enhances data quality.
This approach improves the reliability of imaging endpoints, making them more robust for statistical analysis. Moreover, central imaging plays a key role in supporting regulatory submissions by ensuring the integrity, accuracy, and traceability of data.
With a centralized system, you can confidently present consistent, high-quality imaging results that meet the stringent requirements of regulatory authorities.


非常抱歉,您只有购买软件后才能查看完整软件教程!